Abstract
Background:
The introduction of all-trans-retinoic-acid (ATRA) and arsenic trioxide (ATO) has changed acute promyelocytic leukaemia (APL) from a highly fatal malignancy to a major success in the sphere of haemato-oncology, with long-term survival exceeding 90% in some studies [1]. While literature from the developed world boasts of outstanding outcomes of patients with APL, there is a paucity of data on management strategies, complications and outcomes from the developing world in general and India in particular [2]. With a large population residing in rural areas, high infection rates and limited access to healthcare, we aim to delineate the challenges faced in curing APL in the developing world.
Methods:
We retrospective collected data from medical records of all patients diagnosed with APL between January 2016 to December 2020 in the department of Hematology. A presumptive diagnosis of APL was made based on the presence of abnormal promyelocytes in peripheral blood or bone marrow and flow cytometry (CD13, CD33 positive, HLADR, CD34 negative). The diagnosis was confirmed by the presence of PML-RARα fusion gene by RT-PCR. Data on baseline characteristics, therapy, complications and outcome was collected. Overall survival (OS) was defined from the date of diagnosis to death or date of last follow up. Disease free survival (DFS) was defined from the date of first remission to date of first relapse, death or date of last follow up.
Results:
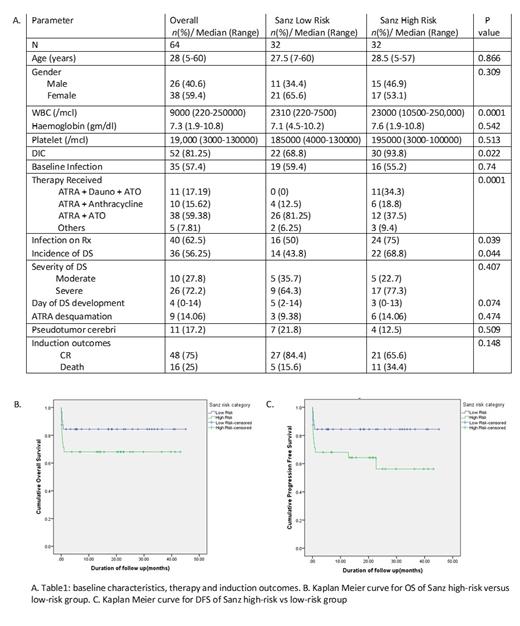
64 patients were treated during the study period. Baseline characteristics, therapy administered and induction outcomes are mentioned in Table 1.
Complications:
56.25% patients developed differentiation syndrome (DS). The incidence was higher in Sanz high-risk group as compared to Sanz-low risk group (68.8% vs 43.2%, p value 0.044).
The incidence of infection at presentation was similar in Sanz high-risk and low-risk groups (55.2% vs 59.4%, p value 0.7). The incidence of new episode of febrile neutropenia during therapy was 62.5% and was higher in Sanz high-risk group as compared to low-risk group (75% vs 50%, p value 0.039). On multivariate analysis, development of infection during induction was a significant predictor of death or relapse (p value 0.02 HR:0.3; 95% CI 0.12-0.84).
17.2% patients developed pseudotumor cerebri. All of them were treated with acetazolamide, 6 patients' symptoms persisted despite acetazolamide and the dose of ATRA was lowered to 25mg/m2.
3 patients developed thrombosis. One patient had superior sagittal sinus thrombosis at presentation and two patients developed central line related thrombosis during therapy.
15.6% patients developed hepatotoxicity of which 3 had an underlying chronic liver disease. The remaining had a normal liver echotexture on ultrasonography and viral markers were normal; the liver dysfunction was attributed to drug induced liver injury.
One patient who received AIDA protocol developed PSVT and improved with adenosine. Another patient who received ATRA+ATO+Daunorubicin developed accelerated hypertension and was managed symptomatically.
Outcomes
75% patients attained complete remission after induction. 9 patients died from infection, 6 from bleeding and one from DS.
At a median follow up of 26 months, 2 patients belonging to high-risk group had relapsed. They received reinduction with ATRA and ATO; one succumbed to pancreatitis during induction, the other attained CR2 and is on maintenance therapy.
The 4-year DFS and OS was 71.25% (95% CI 59.9 to 82.5) and 73.13% (95% CI 62.2 to 84.1) respectively. Patients in Sanz low risk group had a better 4-year DFS (84.9% vs 58.3%, p value 0.026) and OS (84.9% vs 62.5%, p value 0.044) as compared to the Sanz high-risk group. There was a trend towards improved outcomes in high-risk patients with the use of ATRA+ATO+Daunorubicin.
Conclusion:
APL patients in India have a higher incidence of DS, pseudotumor cerebri, hepatotoxicity, baseline infections & new infections during induction complicating therapy. CR rate, DFS, OS were comparable to other population-based studies. ATRA, ATO and daunorubicin combination is the preferred protocol for treating high-risk patients.
REFERENCES:
1. Iland HJ et al. All-trans-retinoic acid, idarubicin, and IV arsenic trioxide as initial therapy in acute promyelocytic leukemia (APML4). Blood. 2012;120(8):1570-80
2. Lo-Coco F et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369(2):111-21.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal